Prosthetic Testing
ATS FoC provides prosthetic testing according to ASTM and ISO standards. A prosthesis is a medical implant designed to replace a missing body part after traumatic injury, degradation, surgical removal, or at birth. Prosthetic designs can differ significantly based on the patient’s needs. Some patients require temporary prosthetics, while others need prosthetics capable of maintaining mechanical integrity throughout long-term use.
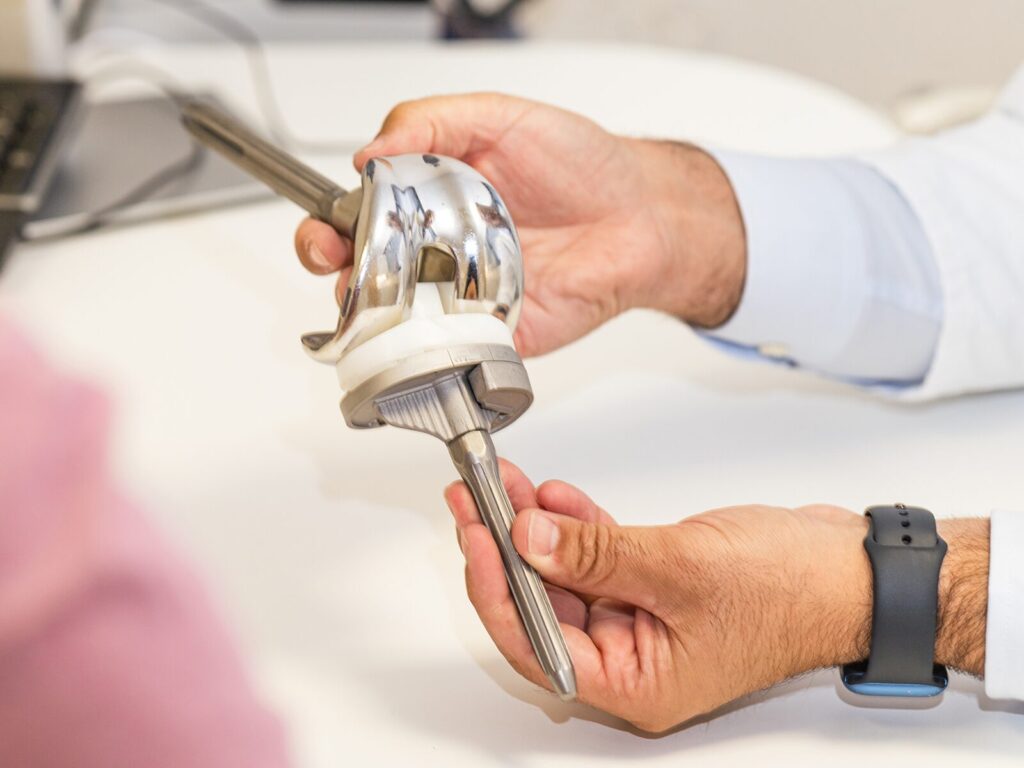
The ATS FoC tests prosthetics for the spine, extremities, joints, and mouth, such as:
- Finger joint replacement
- Glenoid locking mechanism
- Hip systems
- Patellar joint replacement
- Shoulder joint replacement
- TMJ joint replacement
- Total ankle systems
- Total artificial disc
- Total knee replacement
- Total wrist replacement
Why Do You Need to Test a Prosthetic?
The FDA heavily regulates which medical devices reach the market. To meet requirements for legal distribution, manufacturers hire third-party medical device testers such as the ATS FoC. A qualified third-party lab helps clients deliver their products to the market faster through efficient testing recognized by regulatory bodies. Our work helps ensure a prosthetic’s safe and reliable function.
In vitro testing subjects the prosthetic to isolated loads, stress, movements, and environmental factors resembling conditions in a body. We perform custom, non-standard, fatigue, and mechanical testing:
- Axial Compression
- Fatigue
- Instrument and life cycle
- Lot verification
- Shear
- Tensile
- Torsion
- Wear and Simulation
Standards-Based Prosthesis Testing
Our experts can design test methods to ASTM or ISO standards:
- ASTM F1378
- ASTM F1672, Section 6.3.1
- ASTM F1714
- ASTM F2009
- ASTM 2423
- ASTM F2694
- ASTM F2790
- ISO 10328
- ISO 18192-1
- ISO 7206-4, 6, and 8
To learn more about our orthopedic implant testing, click here.
About the ATS FoC
The ATS FoC is a nationwide network of consulting engineering, calibration, testing, and inspection firms. We operate multiple ISO/IEC 17025:2017-accredited labs for medical device testing. Known for our high-quality customer service and thorough analysis, the ATS FoC helps clients make informed business decisions in a variety of industries. Our extensive capabilities enable us to meet our clients’ unique needs quickly and efficiently. In addition to testing in qualified labs, we can provide field services for many tests.
Contact Us
Please call +1 (888) 287-5227 or complete the form on this page to schedule prosthetic testing, and we will help evaluate if your medical device is ready for FDA submission.