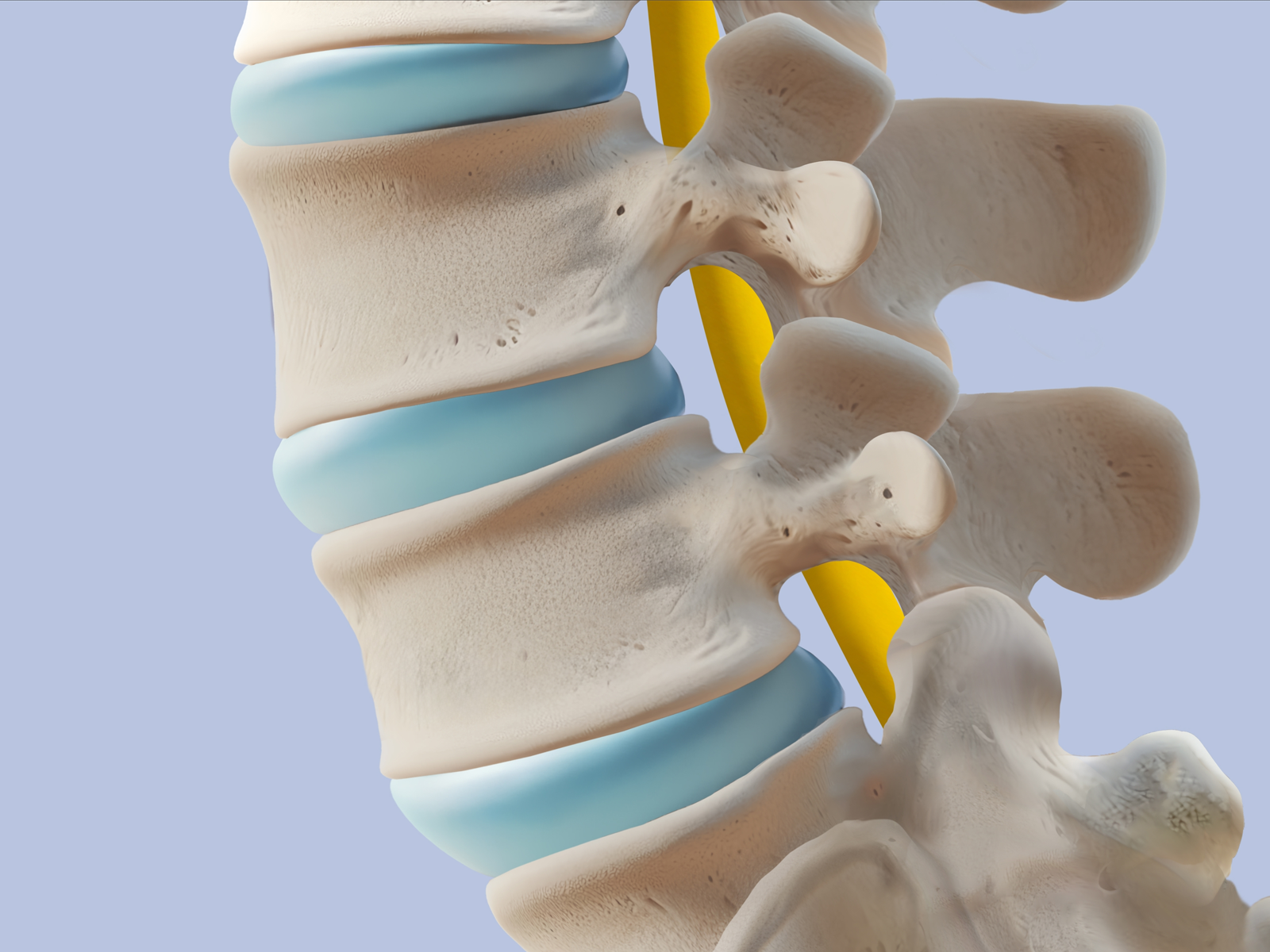
The Applied Technical Services Family of Companies (FoC) tests extra-discal motion-preserving implants in compliance with the ASTM F2624 spinal implant dynamic testing standard. Medical professionals use spinal implants to treat various back pains and deformities, including those caused by degenerative disc disease, fractures, and scoliosis. Our medical device testing facilities assist clients as they qualify their products for the marketplace.
What is ASTM F2624?
The American Society for Testing and Materials established ASTM F2624 as a guideline for technicians conducting dynamic, static, and wear tests on extra-discal motion-preserving implants. The ASTM F2624 standard includes several testing procedures that evaluate the mechanical characteristics of spinal constructs, including the following:
- Fatigue Test
- Static Extension Test
- Static Flexion Test
- Static Lateral Bending Test
- Static Torsion Test
The procedures detailed in ASTM F2624 assist manufacturers in evaluating their designs, ensuring their devices can endure the loading conditions associated with the human spine.
About Our Medical Device Testing Lab
Medical device testing labs offer essential services that ensure the effectiveness, quality, and safety of medical devices that aid healthcare practices worldwide. Our rigorous testing procedures ensure that our clients’ medical devices perform as expected and abide by the standards established by the medical industry’s regulatory bodies. Our medical device testing lab ensures our clients’ satisfaction by providing the following:
- High Quality & Precise Data Collection
- Prompt Services
- Competitive Prices
- ASTM, ISO, & FDA Compliance
Our Commitment to Quality
The Applied Technical Family of Companies (FoC) provides high-quality consulting engineering, inspection, and testing services. Our A2LA accreditation facilities offer an extensive list of services that benefit various industries, including:
- Aerospace
- Automotive
- Chemical
- Communications
- Commercial Properties
- Construction
- Consumer Products
- Defense
- Manufacturing
- Nuclear
- Power Generation
- Renewable Energy
Please submit +1 (888) 287-5227 or submit a web request for additional information regarding our ASTM F2624 spinal implant dynamic testing services.