ASTM F2077 Intervertebral Body Fusion Device Testing
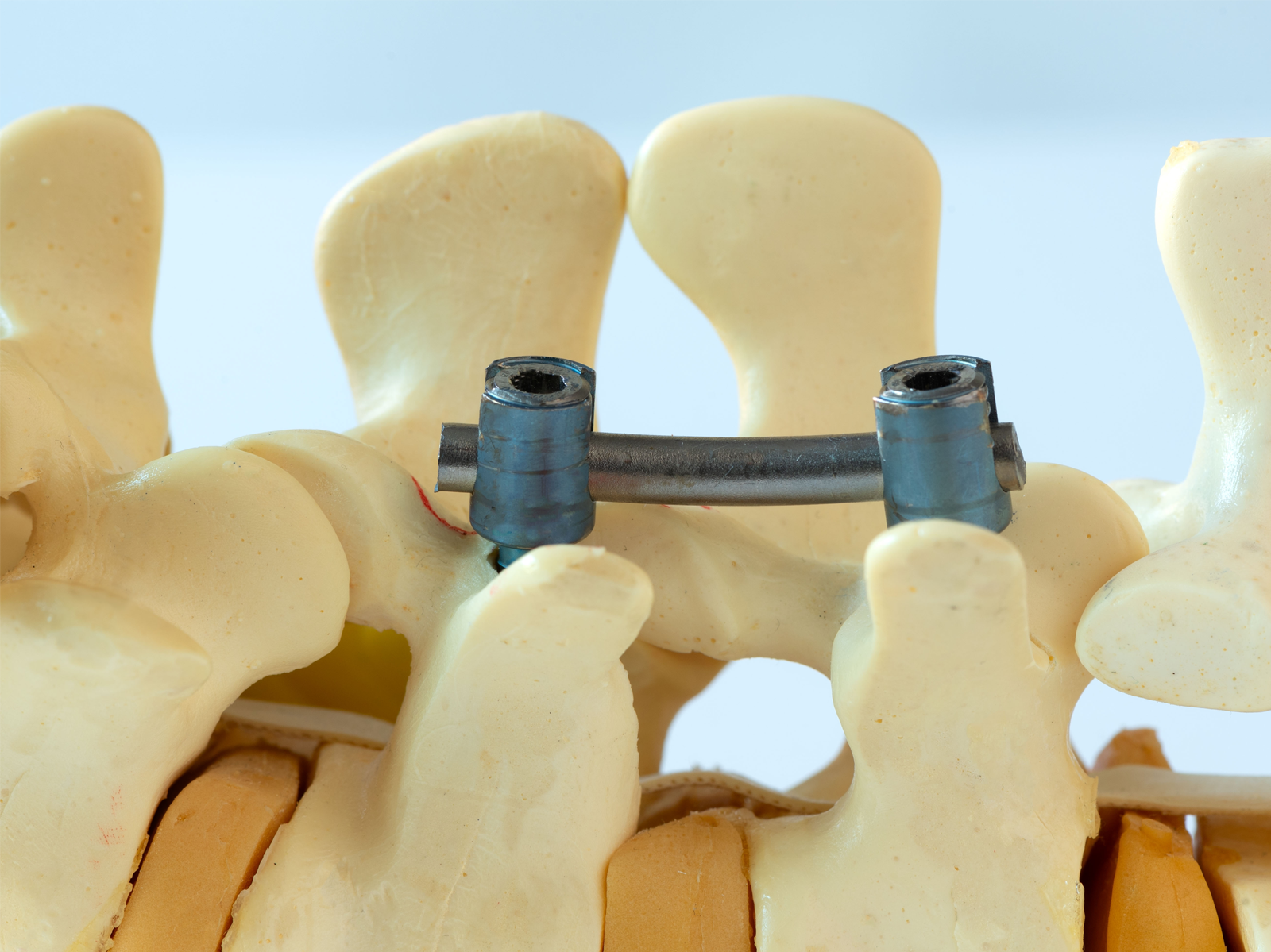
The ATS Family of Companies (FoC) is the ideal choice for manufacturers in need of ASTM F2077-compliant intervertebral body fusion device testing. An intervertebral body fusion device is an implant designed to fuse with the surrounding vertebrae over a year, allowing the adjacent vertebrae and device to operate as a single unit. The device aims to reduce pain upon moving due to injury or degenerative disc disease. People who may benefit from implants live with conditions such as scoliosis, spinal stenosis, spondylolisthesis, and osteoarthritis.
What is ASTM F2077?
Before the FDA approves a product for in vivo testing, the manufacturer must demonstrate the product can hold up to the forces and loads required for daily spinal activity. One method of proving a product’s performance is documented compliance with the FDA-recognized standard ASTM F2077.
Our clients may request ASTM F2077 testing for multiple reasons, such as:
- Confirming proper function
- Testing if the product can withstand the required forces
- Evaluating compliance with FDA criteria
- Comparing different product designs
Intervertebral Body Fusion Device Testing Procedures
Following ASTM F2077 guidance, we perform repeatable static and dynamic tests to determine the causes of deformation and failure. Static tests measure performance under compressive, shear, and torque forces emulating the forces and movements in daily spinal activity. We record displacement, yield, force or moment, and stiffness for each tested subject.
During fatigue tests, the product undergoes varying loads until a functional failure occurs or the product endures 5,000,000 cycles without failing. Our experts use regression analysis to determine the relationship between the product and the applied loads and failure mode (if applicable). We can also incorporate a saline environment into dynamic testing and provide ambient air tests for comparison.
Quality Medical Device Testing from ATS FoC
The ATS Family of Companies (FoC) maintains multiple ISO 17025-accredited labs performing a variety of mechanical and chemical standards. Our comprehensive tests and thorough analyses empower national and international businesses in industries such as medicine and manufacturing. We are committed to providing clients with precise data to support critical decisions and regulatory compliance.
Please call +1 (888) 287-5227 or complete the form on this page to request medical device testing according to ASTM F2077 and other standards.