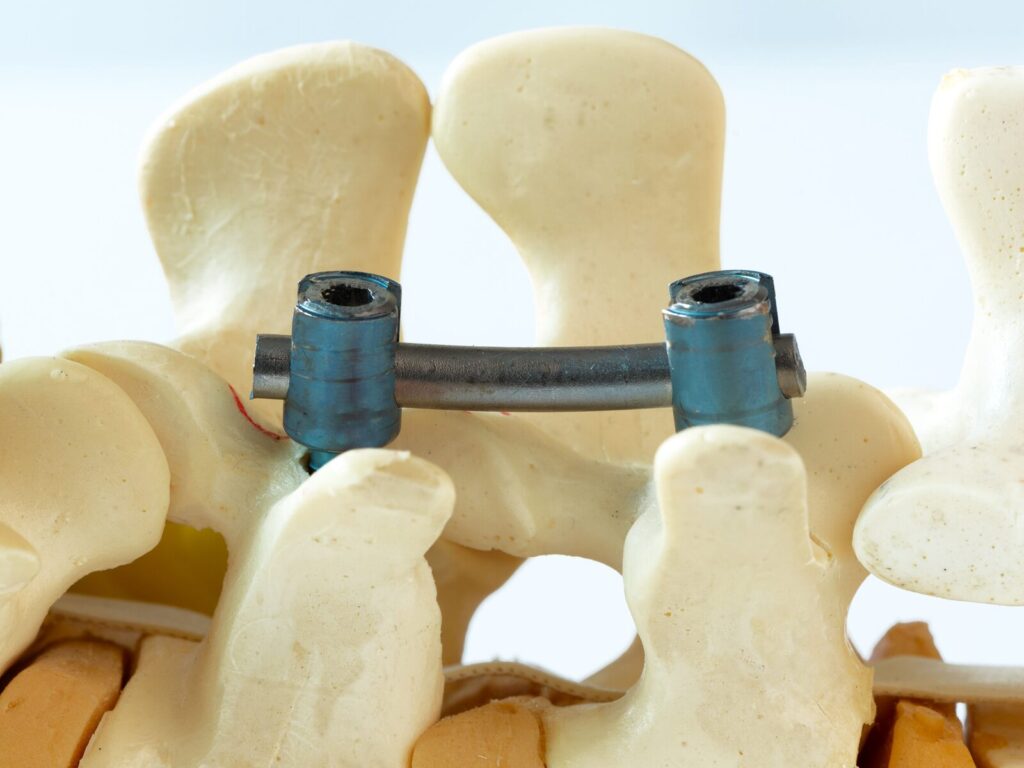
ISO 12189 Fatigue Testing of Flexible Spinal Implants
The ATS Family of Companies (FoC) runs qualified medical device testing labs capable of evaluating spinal implant compliance with industry standard ISO 12189. The International Organization for Standardization (ISO) created standard 12189 for fatigue and biomechanical fatigue testing on flexible spinal implants. Unlike similar standards, the ISO standard considers the influence of anterior support on motion-preserving or vertebral fusion devices.
Why Choose ATS?
ATS FoC testing can enable clients to make superior products. Fatigue testing helps determine the strength of the spinal implant, so manufacturers can decide whether to alter the design or submit the product for FDA approval.
Before permitting in vivo testing, the FDA may require proof from a qualified lab that the device handles in vitro conditions. Poor implant design can cause pain and impact neurological function in the user. As an ISO/IEC 17025:2017-accredited provider of medical device testing, the ATS FoC can deliver an unbiased and reliable assessment of the implant’s performance under cyclic loading.
Fatigue Testing
A fatigue test simulates the cyclic loads a product will encounter in the spine. To represent vertebrae and intervertebral discs in the intended spinal location, we use UHMWPE testing blocks and springs for test repeatability.
Our fatigue testing equipment can deliver loading and unloading forces at a set frequency until the machine completes 5 million cycles or fatigue failure occurs. Fatigue failure arises when a load initiates a crack in the device that propagates until the assembly fractures.
Our powerful equipment can deliver loads that simulate worst-case scenarios and the repeat forces experienced in daily life:
- Load capacity up to 55 Kip
- Additional environmental controls, such as temperature from ambient to 1,000˚ C
We use fatigue testing to calculate the static and dynamic strength of the spinal implant by measuring how many cycles of a certain cyclic load until permanent deformation. This quantitative analysis helps manufacturers anticipate a product’s reactions to a variety of loading scenarios.
About ATS FoC
The ATS FoC operates multiple locations nationwide, allowing for more speedy and convenient shipping for laboratory testing or in-person inspections. Our experts use their decades of combined experience in medical device testing for thorough tests and analysis.
We hold ISO/IEC 17025:2017 accreditation to perform the following:
- Mechanical testing
- Nondestructive testing
- Chemical testing
- Electrical testing
- Calibrations
Call +1 (888) 287-5227 or complete the request form on this page to schedule ISO 12189 testing today.