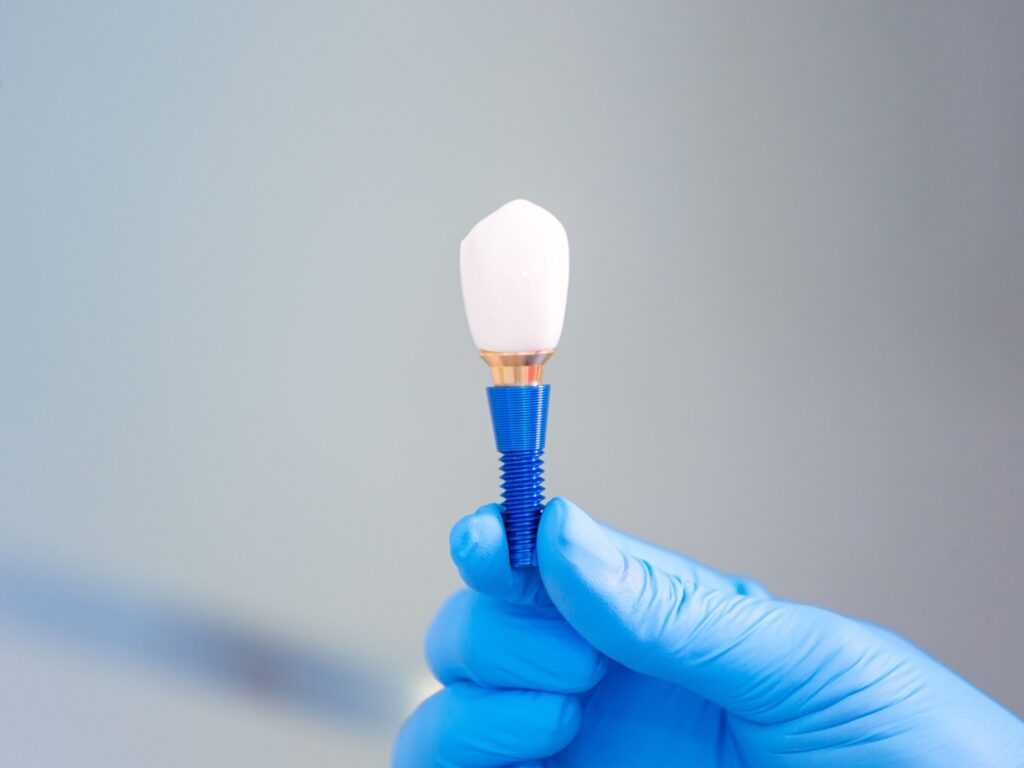
The Applied Technical Services Family of Companies (FoC) determines the fatigue performance of dental implants per the methodology outlined in the ISO 14801 testing standard.
What is ISO 14801?
ISO, or the International Organization for Standardization, established ISO 14801 as a resource for experts evaluating how endeosseous dental implants perform under dynamic loads. The standard simulates the mechanical stresses endured by dental implants, offering critical insight into the implant’s fatigue performance. However, it is not a reliable predictor of in-vivo performance, and it doesn’t apply to implants with endosseous lengths shorter than 8mm nor does it apply to magnetic attachments. The standard is most effective for comparing the performance of transmucosal dental implants of different shapes, sizes, and designs.
About Our Medical Device Testing Lab
Our medical testing laboratory offers a range of tests that assess the behaviors, strengths, and limitations of various medical devices, instruments, and materials. Our specialists work diligently to deliver solutions to clients who are seeking to comply with regulations and clients who have custom testing needs. Our A2LA-accredited facilities feature properly maintained equipment that yields accurate and reliable results, ensuring our clients come away with the data needed to meet their project’s demands. We understand the urgent need to keep medical devices safe and compliant, so we always stay up to date on the latest guidelines, expectations, and industry news to ensure that our services offer long-term solutions. All of our standard-specific tests comply with the preparation and procedural requirements outlined in the standard and we document our process accordingly and supply our clients with a detailed analysis.
Why is ISO-Compliance Important for Dental Implants?
ISO standards are internationally adopted and recognized and while they may not be explicitly required, they are often the consensus standard for the FDA and similar regulatory entities. Therefore, ISO compliance is a critical step in the path toward a medical device’s eligibility to be sold on the market. Consumers and medical providers alike have increased confidence in products that have been assessed in alignment with expectations set by trusted agencies like ISO and ASTM.
Contact Us
For more information about our medical device testing capabilities or to learn about our consulting services, please call +1 (888) 287-5227.