ASTM F2346 Standard and Dynamic Testing of Artificial Intervertebral Discs
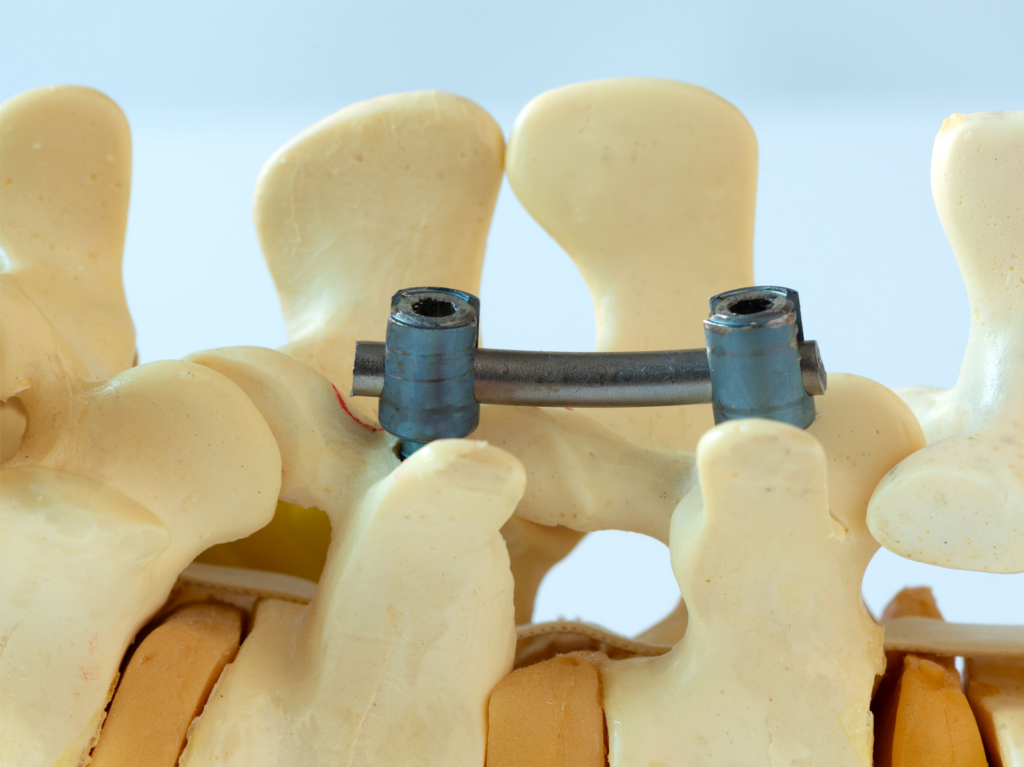
The ATS Family of Companies (FoC) provides medical device testing for artificial intervertebral discs according to the ASTM F2346 standard. Artificial intervertebral discs are orthopedic implants that replace weak or narrowed discs in a patient’s spine. Typically, the orthopedic implant is inserted between two vertebrae in the cervical or lumbar regions.
Artificial discs can absorb the stresses on the vertebrae, reducing back pain and preserving spinal motion in patients with back pain or degenerative disc disease. To ensure the implants retain mechanical integrity in their intended environments, ASTM designed the F2346 standard for in vitro testing.
ASTM F2346: Artificial Intervertebral Disc Testing
The ASTM F2346 standard specifies methods and materials to compare how various artificial intervertebral discs react to static and dynamic loads. These tests subject the orthopedic implants to various conditions that simulate the shock and stress demanded by regular motion.
Artificial intervertebral discs typically consist of two keel or spike endplates and a core. Most FDA-approved orthopedic discs are made from a combination of plastic, metals, and coatings, such as:
- Cobalt chromium alloy
- Molybdenum
- Hydroxyapatite
- Titanium alloy
- Polyethylene
Types of Tests
Per ASTM requirements, the ATS FoC can conduct the test in a 0.9% saline bath at 37°C to simulate the temperature and moisture of the vertebral cavity. We can use the same equipment throughout the procedure, making minor adjustments depending on the test:
- Axial compression
- Compression shear
- Torsion testing
Static Tests
First, we determine the maximum load the product can endure. Next, we perform tests with compression, shear, and torque forces. During a torsion test, we apply physiological compression preloads based on the intended location of the artificial disk, with 100 N for the cervical region and 500 N for the lumbar region. Finally, we record variations in load, displacement, moment, and angle as needed.
We can calculate stiffness, yield displacement, yield load/moment, ultimate yield load/moment, and ultimate displacement.
Dynamic Tests
Our experts can also conduct dynamic tests to assess performance under changing conditions at a cyclic frequency of 2Hz. Using the same testing apparatus, we can subject the product to various fatigue loads or moments until the test reaches 10,000,000 cycles or functional failure occurs. We can calculate the endurance limit of the artificial disc through regression analysis and note the type of failure, failure mode, and any deformation or mechanical deterioration.
Our Commitment to Quality
The ATS FoC operates multiple ISO 17025-accredited labs nationwide. With a wide scope of accreditations in medical device testing, we are uniquely qualified to provide clients with dependable solutions.
Our experts use innovative technology and follow testing procedures outlined by regulatory and industry authorities to deliver comprehensive testing and analysis. Our quick turnaround rate gets products to market faster and helps clients make critical business decisions sooner.
Contact Us
Please call +1 (888) 287-5227 or submit an online request to schedule our artificial intervertebral disc testing services.