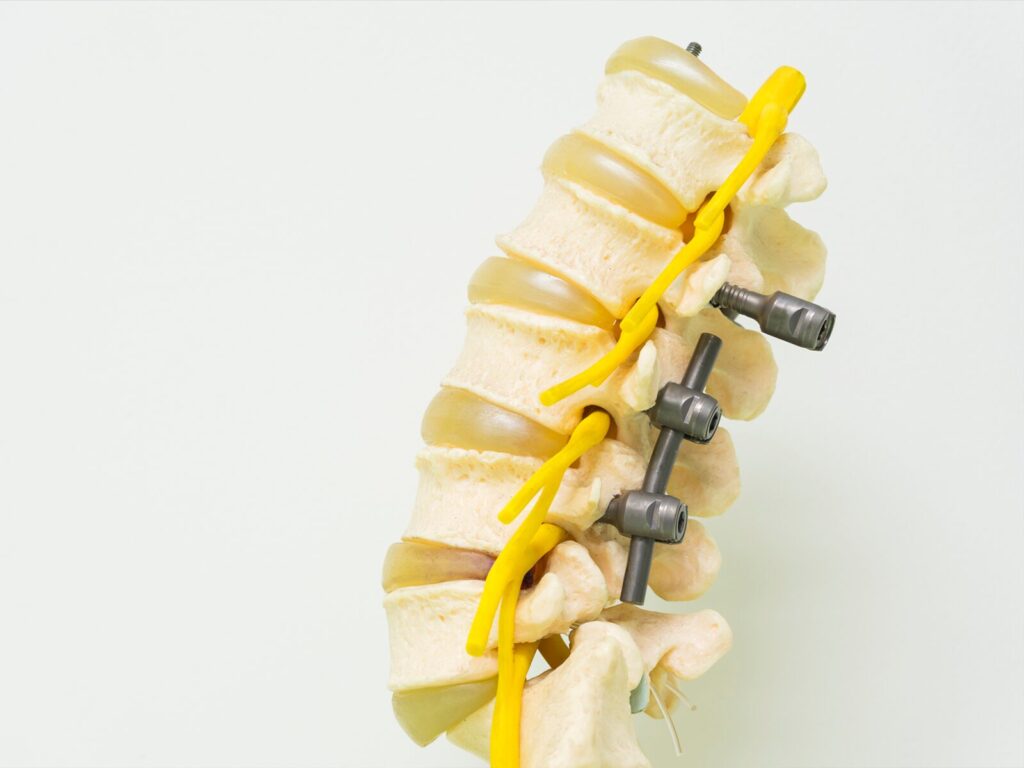
The Applied Technical Services Family of Companies (FoC) evaluates spinal implant constructs in compliance with the ASTM F1717 spinal implant testing standard. Spinal implants help stabilize spines that have suffered damage from injuries, chronic illnesses, and spinal disorders. Our medical device testing lab helps our clients ensure the quality of their spinal implants and qualify their products for the marketplace.
What is ASTM F1717?
ASTM F1717 outlines the materials and methods necessary to conduct fatigue and static tests in spinal implant assemblies in a vertebrectomy model. The procedure allows technicians to compare the mechanical characteristics of past, present, and future spinal implants. This testing applies to a range of spinal devices, including cervical and lumbar plating systems.
Before the procedure, technicians load the plates or pedicle screw systems onto a pair of test blocks comprised of ultra-high molecular weight polyethylene (UHMWPE). Next, the technicians subject the sample to dynamic and static loads.
ASTM F1717 outlines the following three static tests to quantify sample performance:
- Compression Bending
- Tension Bending
- Torsion Testing
Our Medical Device Testing Lab
Medical devices are essential to the efficiency and effectiveness of modern healthcare, and our medical device testing lab ensures that these perform as expected and adhere to FDA and ASTM regulations and standards. We offer an extensive list of ASTM-compliant implant testing services, including spine, dental, joint, and extremity testing.
About the ATS FoC
The ATS FoC provides high-quality inspection, testing, and consulting engineering services to industrial and commercial clients in various industries. Our numerous ISO/IEC 17025: 2017 (A2LA) accredited facilities assist clients with their chemical, mechanical, electrical, and nondestructive testing, and calibration needs.
High-Quality Services
We adhere to internationally recognized standards of quality to ensure that our clients receive world-class service from each of our facilities. Our experienced and informative employees work closely with clients to develop strategies and procedures that directly address their unique needs. We value our client’s time, so we provide professional service in a timely manner without compromising our commitment to industry-leading accuracy and quality.
Contact Us
To inquire about ASTM F1717 spinal implant testing services, call +1 (888) 287-5227 or submit an online request form.